The Green Zinc Project focuses primarily on recovering zinc—and associated metals such as lead—from secondary and waste materials generated in metallurgical industries. Traditionally, fossil-based reductants such as coal, petroleum coke, and metallurgical coke are used in these processes; however, their use contributes significantly to anthropogenic CO₂ and other greenhouse-gas (GHG) emissions. Biochar offers a sustainable alternative. Produced via biomass pyrolysis in an inert atmosphere at elevated temperatures, biochar is considered CO₂-neutral and contains high fixed-carbon levels comparable to those of fossil reductants.
Despite its advantages, raw biochar presents challenges for metallurgical applications due to its low bulk density, high reactivity, porous structure, and poor mechanical strength. To address these limitations, surface modification and densification were performed using various binders, including bentonite, calcium hydroxide, cement, molasses, and lignosulfonate. These binders promote pellet hardening by altering the capillary and viscous forces bonding individual biochar particles.
A more advanced densification method, microgranulation, was also applied. This well-established technique increases pellet grain size, density, and mechanical strength while decreasing reactivity. During microgranulation, binders fill and close the macropores within the biochar matrix, thereby reducing its reactivity and enhancing robustness. The effectiveness of the surface modification was evaluated using scanning electron microscopy (SEM) to characterize morphological changes and a modified coke reactivity index (CRI) to assess reactivity. SEM images (Figure 1) confirmed successful modification of the biochar surface, while the combined SEM and modified CRI results were used to evaluate the overall success and performance of microgranulation as a surface-modification technique.

Figure 1: Microstructure of untreated biochar, petroleum coke for comparison and modified biochar with 5 wt.% bentonite
A modified Coke Reactivity Index (CRI) test was performed using a tube furnace. Heating to 950°C took place at a heating rate of 6.3 K/min under a nitrogen atmosphere. After reaching the targeted temperature, it was held for 15 mins in a CO2 atmosphere supplied at 5 L/h. The addition of binders significantly reduced the reactivity of biochar as shown by Figure 2.
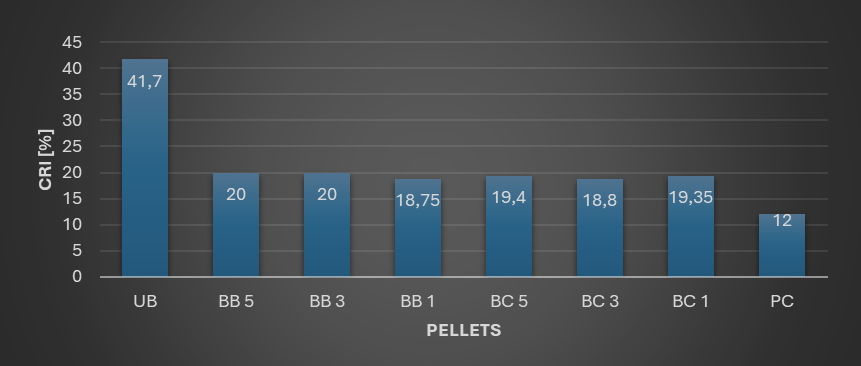
Figure 2 : Modified CRI values of biochar samples (UB: untreated biochar, BB1: biochar-1wt.% bentonite, BB3: biochar-3wt.% bentonite, BB5: biochar-5wt.% bentonite, BC1: biochar- 1wt.% Ca(OH2), BC3: biochar- 3wt.% Ca(OH2), BC5: biochar- 5wt.% Ca(OH2)and PC:petroleum coke)
Impacts and effects
Microgranulation proved to be an essential and effective pretreatment method for surface-modifying biochar, leading to improved mechanical strength and reduced reactivity suitable for metallurgical applications. In its untreated form, biochar is low-density and highly reactive; consequently, it tends to combust rapidly when exposed to hot gases in pyrometallurgical processes, preventing effective participation in melt-phase reduction. Through microgranulation, the reactivity of biochar was significantly reduced, slowing its instantaneous combustion and enabling sufficient residence time for the desired reduction reactions within the melt. The success of this process contributed directly to the development and filing of a patent.